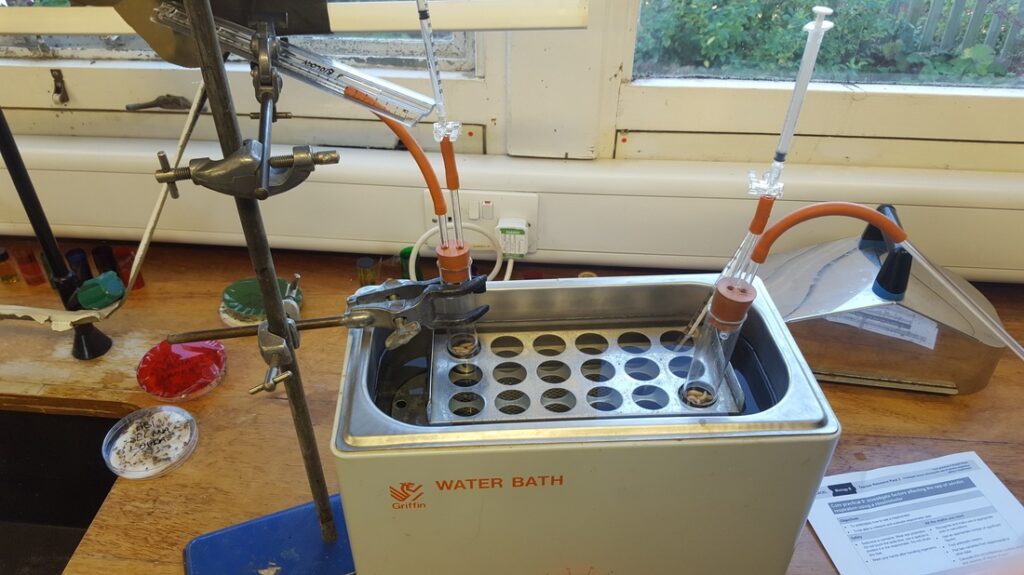
Objectives
- To understand how to use a respirometer
- To be able to interpret and evaluate respirometer data
Safety
- Soda lime is corrosive. Wear eye protection, do not touch the soda lime; use a spatula to position it in the respirometer and do not inhale any dust
- Wash your hands after handling organisms
Equipment
- Respirometer
- Live animals (10x maggots)
- Soda lime in muslin
- Coloured manometer fluid
- Spatula
- Stop clock
- Clamp and stand
- Dropping pipette
- Eye protection
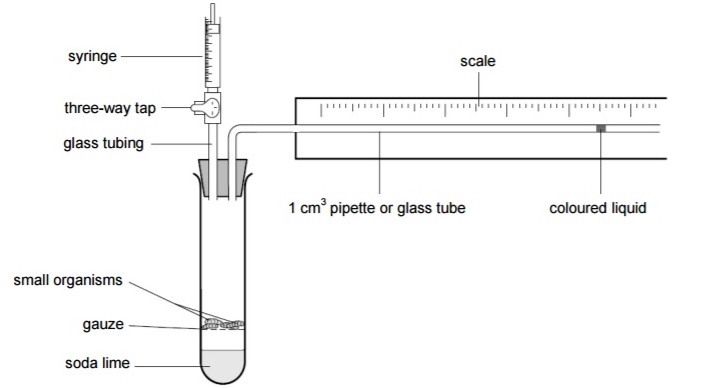

Method
1.Assemble the respirometer like the diagram, check that you know how to use it, especially the operation of the three way tap. Clamp the syringe and the respirometer in position when in use
2.Place as known mass of one type of organsm into the boiling tube. For our experiment we used 10 maggots, it is still accurate to use a quantity as the maggots were generally uniform in size so the mass would have been similar. Handle live animals with care to avoid harming them]
3.Place a drop of coloured fluid at the open end of the glass tube using the dropping pipette. Open the connection between the syringe and the respirometer. Use the syringe to draw the fluid onto the scale at the end furthest from the respirometer
4.Mark the starting position of the fluid
5.Close the tap to isolate the respirometer from the atmosphere and the syringe and start the stop clock immediately
6.Note the position of the fluid at one minute intervals for at least five minutes
7.Work out the distance travelled by the liquid during each minute. Record your results in a suitable table.
Results
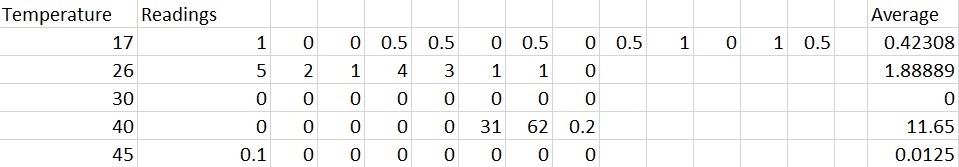
Above is the class results. Using the mean on right of table it is possible to plot a graph with Temperature (Celsius) on the x axis and mean on the y axis.
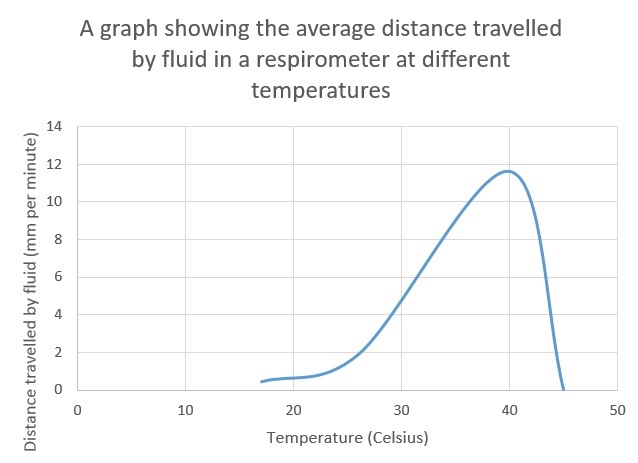
From the table we can see the reading for 30 degrees is most likely to be anomalous as the reactions before it displayed some respiratory activity. The one after it has increased drastically showing that we should have had results at 30 degrees and because of this I have excluded it from the curve of best fit. The reason we have no results for this data set is perhaps due to improper use of equipment or an air gap somewhere which allowed the maggots to respire in an open system causing the coloured liquid to not move.
Analysis of results
For our experiment we can see there is a clear optimum temperature at about 40 degrees. The reason there is a definite optimum and no reading at other temperatures (45 degrees and above) is because respiration is an enzyme controlled reaction. All enzymes will denature above a certain point as an enzyme’s active site can be changed by temperature as quaternary structure will be altered. This can mean at certain temperatures the substrate molecule cannot fit into the active site. This would mean that no reactions would occur. At a temperature such as 20 degrees where some respiration occurs, but not as much as at 40 degrees this is because the temperature changes the active site, not enough to not let the substrate molecule bind to the active site but enough that the rate is significantly reduced.
Evaluation of results
As discussed in our analysis there is clearly an optimum temperature however our value is not necessarily accurate as we used temperatures that had irregular large intervals between them. This means that the optimum temperature, despite in our experiment being observed at 40c , is closer to 35 degrees. If we were to repeat the experiment, we could increase the number of temperatures at which we taken readings at, for example every 5 Celsius and repeat the experiments and find a mean across multiple experiments. This would provide us more data points and give us a more accurate optimum temperature and points at which the enzyme denatures. We can compare out experiment to another set of data found online.[2] It was a similar experiment with significant differences being that mass of organism was used and that the researcher noted that with his equipment there was a potential for an air leak and that ‘some of the maggots crawled into the soda lime… resulting in their death’. As a non-uniform number of maggots would have died per experiment we can expect a certain level of inaccuracy but at the same time it would still be valid to draw comparisons. Below is the table I constructed from the data gathered from the experiment, this particular experiment was recorded every 5 Celsius. The student estimated that the graph was approximately 10 degrees lower than it should have been through experimental inaccuracies and due to the Q10 law that states the reaction should have increased until 40 degrees[3]. We can see that both graphs have a similar distribution and our graph follows the Q10 law. This shows that our experiment is relatively accurate.
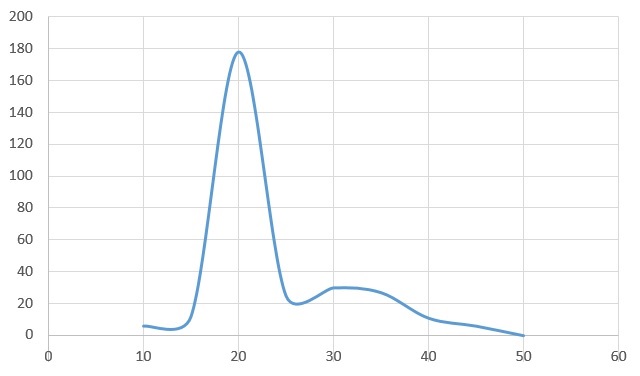
An error that occurred in our experiment was that at the highest temperature of 45c the maggots after a period of time died due to the high eat and being unable to respire. This shows that the maggots cannot respire at a high temperature so as to not be cruel to the living organism, we would not repeat this experiment and the result would be the same again.
Questions
- Animals usually have a higher respiration rate per gram than plants. Explain this difference.
- An animal is generally larger with more complex body functions and is, generally, moving throughout the day. The muscles which move throughout the day require a constant supply of energy whereas plants are stationary. Plants require very little energy compared to animals for metabolic (breaking down) reactions within the organism
- Suggest what factors may have caused any variability seen in class results
- Over 5 experiments with separate equipment there is a likely chance that one of the sets of equipment’s may have a leak or a crack in the glass. If this is not noticed and the reaction continues we would see vastly different results to the expected (for example 30c). For our experiment we chose ten maggots and, as mentioned earlier, the maggots are generally a similar weight however it is impossible to have a consistent weight if you are using quantity of organism. If we were to repeat the experiments and got subtly different results this may be the cause
- How could this variability be reduced and the precision of the results improved?
- The variability could be reduced by ensuring the equipment is functioning and set up properly. Use the same equipment for each reaction to ensure no fault is due to equipment. Next time weight the maggots and then from this we can calculate respiration rate per gram of organism which would allow for more analysis
- It would have been better to have used a control respirometer alongside the experimental set-up. In the control the equipment is the same but the organisms are replaced by non-living material such as glass beads
- Explain what may cause the liquid in the control tube to move towards and away from the respirometer
- As the gas inside the respirometer heats up its volume increases, this is known as thermal expansion
- Explain how you would use the control results to correct your experimental data
- If at 40c we find that the liquid naturally moves 2mm due to thermal expansion, we can add 2mm to the distance moved by the fluid as we would not have noticed or observed this decrease as the reaction was occurring but the maggot’s respiration would have had to ‘overcome’ this 2mm of gaseous debt
- Explain what may cause the liquid in the control tube to move towards and away from the respirometer
- What is the importance of using soda lime in the respirometer? How does this affect the volume of gas in the apparatus? How does this influence the movement of the liquid in the capillary tube?
- The soda lime is used to absorb the carbon dioxide produced by photosynthesis. If there was no soda lime the oxygen would be converted into oxygen via respiration meaning no actual change to the volume of gas in the apparatus. By absorbing the carbon dioxide, we can measure the amount of oxygen gas used for respiration by measuring the distance the fluid has travelled. The fluid moves to maintain a constant pressure as the volume is decreasing due to the oxygen being used and carbon dioxide then being absorbed