

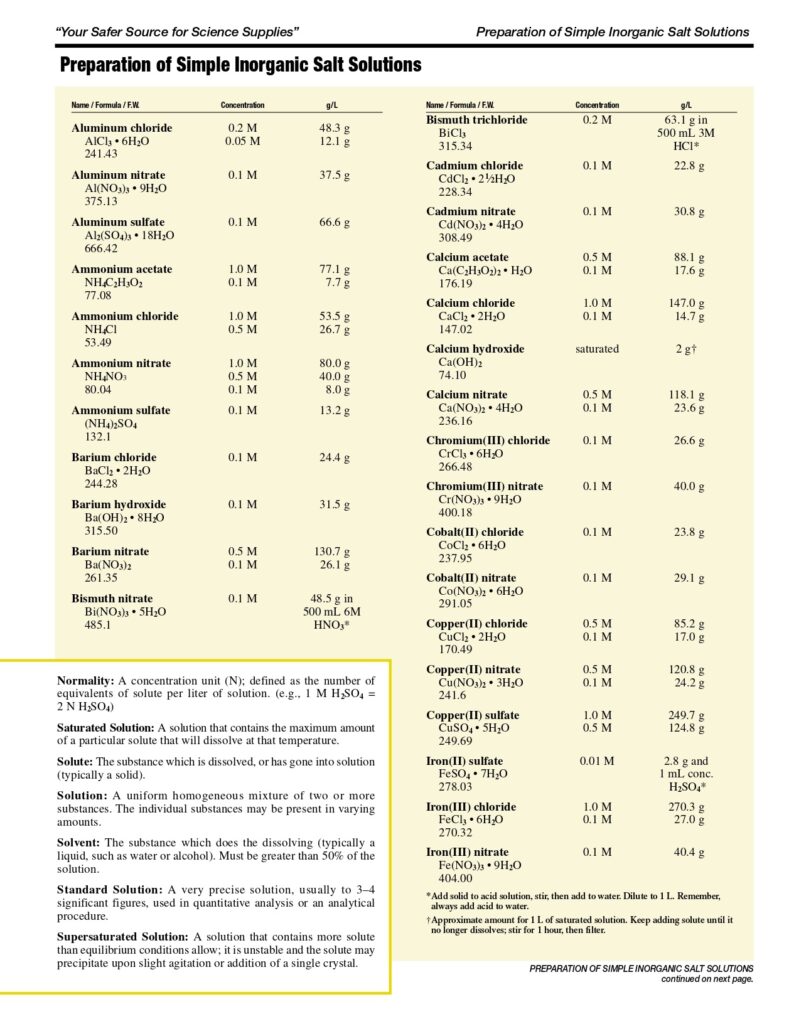

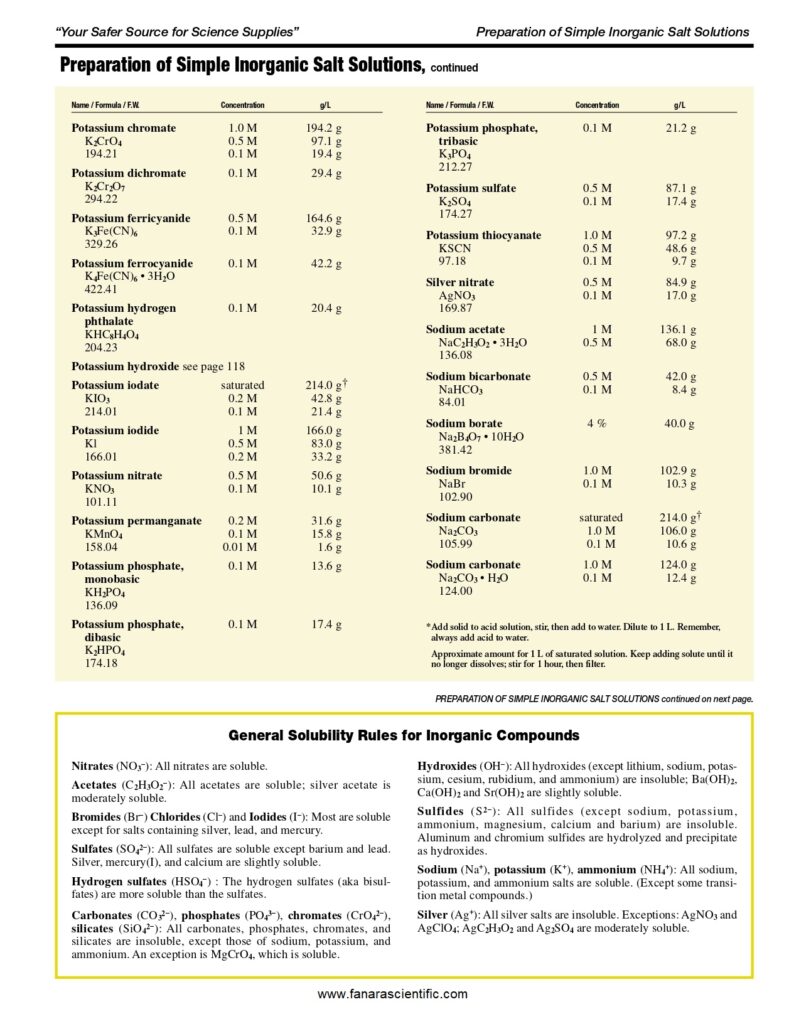
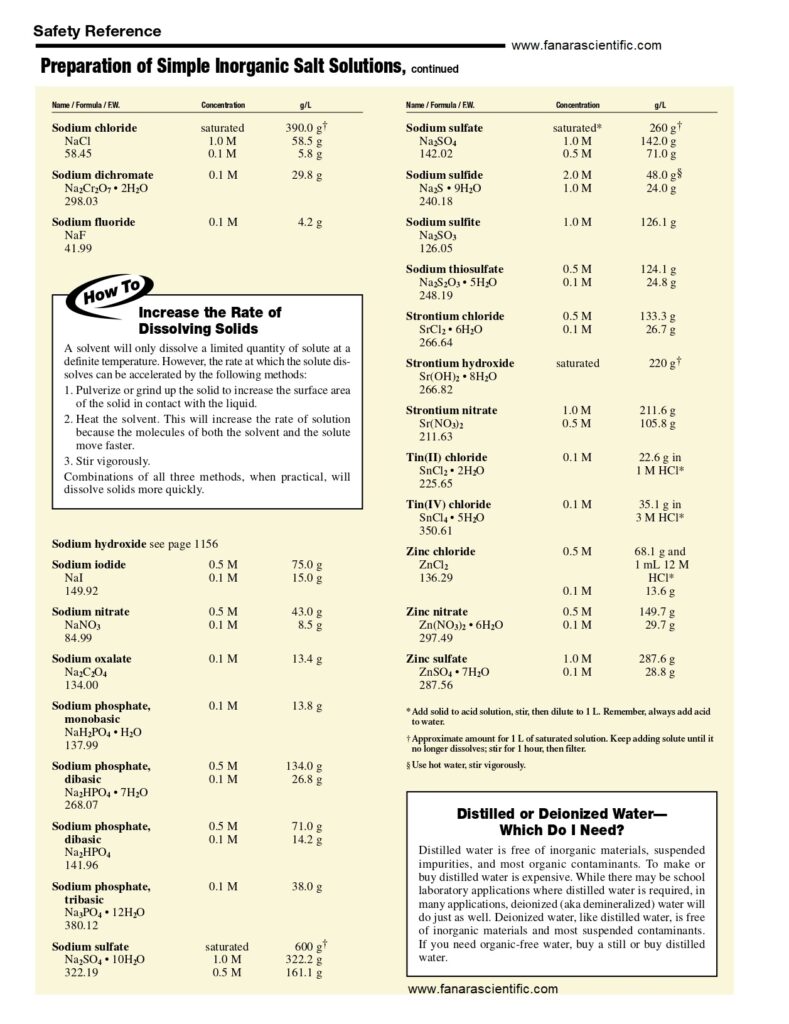









For 1 litre of 1x PBS: phosphate buffer solution
- Begin with 800ml of distilled water in a Duran bottle.
- Measure out and add 8g of NaCl.
- Add 1.44g of Na2HPO4.
- Add 0.2g of KCl.
- Add 0.24g of KH2PO. …
- Bring the pH to 7.4 or 7.2 (depending on the application) using HCl.
- Add distilled water until the total volume reaches 1 litre.
========================================================
How do you make 0.1 M phosphate buffer?
0.1 M phosphate buffer solution pH 6.5.
Dissolve 13.80 g of sodium dihydrogen phosphate monohydrate R in 900 ml of distilled water R. Adjust the pH (2.2. 3) using a 400 g/l solution of sodium hydroxide R.
===========================================================
What is 0.01 M phosphate buffer solution?
0.01 M Phosphate Buffered Saline pH 7.4 is a common buffer extensively used in molecular biology and biochemistry as it can maintain pH and osmolarity. 0.01 M Phosphate Buffered Saline pH 7.4 Solution is supplied as a ready to use form for convenience.












